Senna Syrup 8.8mg/5mL Recall – Jan 11th 2022
Download this notice as a PDF Here
Lohxa LLC Issues Voluntary Nationwide Recall of Senna
Syrup 8.8mg/5mL Due to Microbial Contamination
Company Contact
Name: Jessica Mendoza
Phone Number: 800-641-5564
FOR IMMEDIATE RELEASE – 01/11/2022 – Worcester, MA. Lohxa LLC is voluntarily recalling one lot of Senna Syrup 8.8mg/5mL, unit-dose cups to the consumer level. The product is being recalled due to microbial contamination.
Use of contaminated product by the elderly, patients with a weakened immune system, or patients at a higher risk of developing life-threatening inflammation of the heart, could result in infections that could be life-threatening. To date, Lohxa LLC has not received any reports of adverse events related to this recall.
The product is used as a natural vegetable laxative for the relief of occasional constipation and is packaged into 5 mL unit-dose cups. The product is distributed into cases of 20 cartons packaged with 24 units each, NDC: 50268-731-24. The affected Senna Syrup 8.8mg/5mL lot is AM1115S with expiration date of 01/2023. The product can be identified by the label below. Product was distributed to AvKare (Wholesaler) who may have further distributed this to clinics, hospitals, and healthcare providers.
Outer carton labeling


Unit-Dose Cup
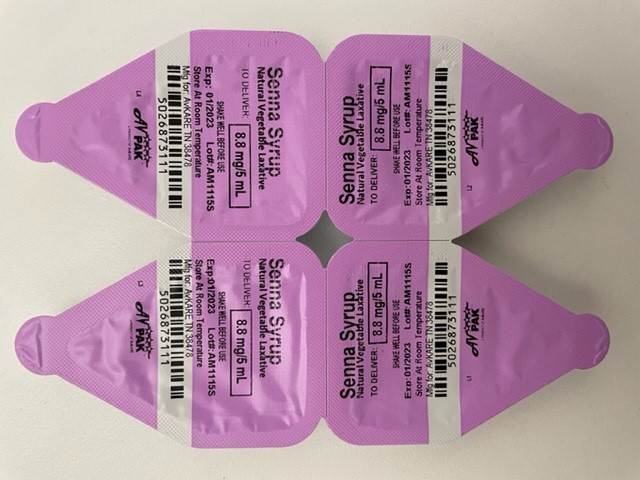
Lohxa LLC is notifying its distributors and customers by letter and is arranging for return of all recalled products. Consumers/distributors/retailers that have product which is being recalled should stop using the product and return it to place of purchase.
Consumers with questions regarding this recall can contact Lohxa LLC by 800-641-5564 or by email to info@lohxadirect.com Monday-Friday from 9am-5pm EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.